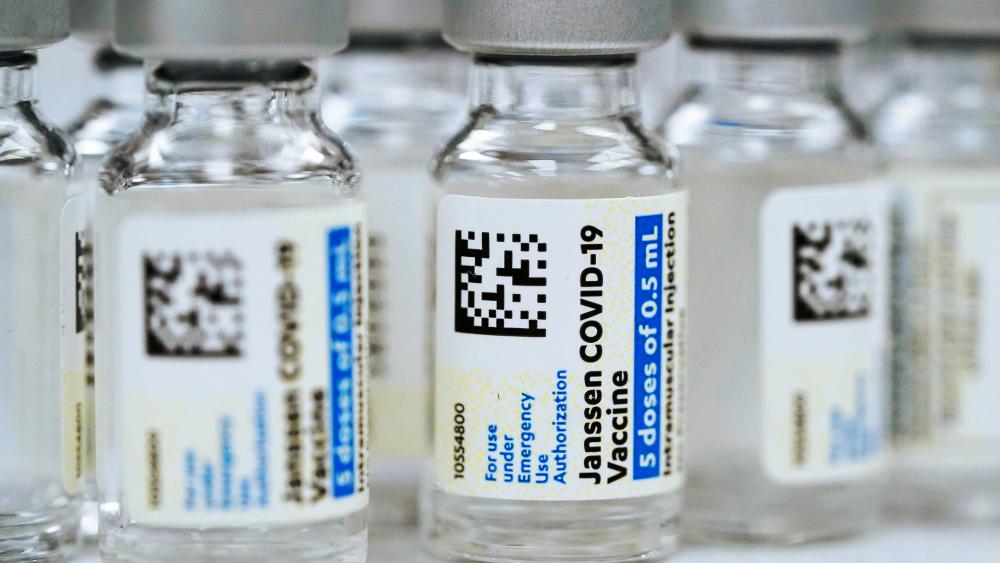
The U.S. government has put strict limitations on the Johnson & Johnson COVID vaccine because of a serious health risk.
Regulators took that action because of the ongoing possibility of rare, but serious, blood clots.
The Food and Drug Administration (FDA) said the shot should only be given to adults who can't get any other vaccine or specifically request the one from Johnson and Johnson. U.S. authorities for months have recommended that Americans get Pfizer or Moderna shots instead of the J&J vaccine.
FDA vaccine chief Dr. Peter Marks said the agency decided to restrict the vaccine after taking another look at the data on the risks of life-threatening blood clots and concluding that they are limited to J&J's vaccine.
"If there's an alternative that appears to be equally effective in preventing severe outcomes from COVID-19, we'd rather see people opting for that," Marks said. "But we've been careful to say that – compared to no vaccine – this is still a better option."
The problem occurs in the first two weeks after vaccination.
So if you got a Johnson & Johnson shot a while ago, and haven't had problems with blood clots, experts say you can rest easy.
CDC Recommended Other Shots
As CBN News reported last December, the Centers for Disease Control and Prevention recommended Moderna and Pfizer shots over J&J's because of the rare blood clotting condition.
At that time, the strange clotting problem caused nine confirmed deaths after J&J vaccinations.
As of mid-March, federal scientists had identified 60 cases of the side effect, including nine that were fatal. That amounts to 3.23 blood clot cases per 1 million J&J shots. The problem is more common in women under 50, where the death rate was roughly 1 per million shots, according to Marks.
Marks said the FDA spent extra time analyzing the problem to be sure it wasn't connected to a separate issue, such as women taking birth control medications that raise their risk of clotting.
The J&J vaccine will carry a starker warning about the potential "long-term and debilitating health consequences" of the side effect.
A J&J spokesman said in an emailed statement: "Data continue to support a favorable benefit-risk profile for the Johnson & Johnson COVID-19 vaccine in adults when compared with no vaccine."
***Please sign up for CBN Newsletters and download the CBN News app to ensure you keep receiving the latest news from a distinctly Christian perspective.***
FDA Authorized J&J Shot in February 2021
The Johnson & Johnson vaccine was first authorized by the FDA in February of 2021 for emergency use for adults 18 and up. It was the first single-shot vaccine in the fight against the global pandemic.
The vaccine was initially considered an important tool in fighting the pandemic because it required only one shot. But the single-dose option proved less effective than two doses of the Pfizer and Moderna vaccines.
The rollout of the company's vaccine was hurt by a series of troubles, including manufacturing problems at a Baltimore factory that forced J&J to import millions of doses from overseas.
However, clotting problems first came up last spring, with the J&J shot in the U.S. and with a similar vaccine made by AstraZeneca that is used in other countries. At that time, U.S. regulators decided the benefits of J&J's one-and-done vaccine outweighed what was considered a very rare risk — as long as recipients were warned.
Potentially Dangerous Neurological Reaction to Shot Reported Last July
As CBN News reported in July of 2021, regulators added a new warning to Johnson & Johnson's COVID-19 vaccine about links to a rare and potentially dangerous neurological reaction but said it was not entirely clear the shot caused the problem.
The FDA announced the warning, flagging reports of Guillain-Barre syndrome, an immune system disorder that can cause muscle weakness and occasionally paralysis. Health officials described the side effect as a "small possible risk" for those getting the shot.
The action came after the FDA and the CDC reviewed reports of about 100 people developing the syndrome after receiving the one-dose vaccine. Almost all of were hospitalized and one person died, the FDA said.
Your health is important. Do you have questions about nutrition, weight loss, boosting immunity or medicine? Learn more here!
Need prayer? We’re available 24/7. Call (800) 700-7000 or request prayer.
Learn why Truth Matters at CBN News.Did you know?
God is everywhere—even in the news. That’s why we view every news story through the lens of faith. We are committed to delivering quality independent Christian journalism you can trust. But it takes a lot of hard work, time, and money to do what we do. Help us continue to be a voice for truth in the media by supporting CBN News for as little as $1.











 Support CBN News
Support CBN News