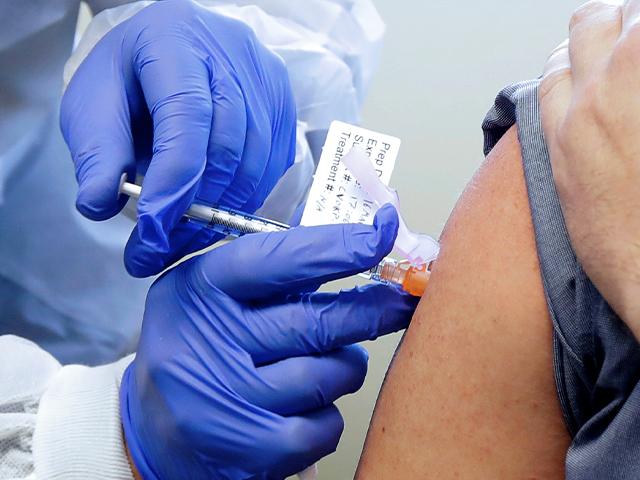
There's been a potential setback in efforts to pursue a COVID-19 vaccine as one of the most promising trials has now been placed on hold.
Researchers are pausing the stage three vaccine trial being conducted by Oxford University in the UK after a volunteer came down with an unexplained illness.
AstraZeneca, the company developing that vaccine with Oxford, is conducting an investigation to determine if the illness is linked to the trial.
In a statement, the company insisted that they won't seek approval or emergency use until "demonstrating safety and efficacy through a phase 3 clinical study.
"The safety and efficacy of vaccines, including any potential vaccine for COVID-19, is reviewed and determined by expert regulatory agencies around the world, such as the United States Food and Drug Administration (FDA)" the statement reads. "FDA has established clear guidance for the development of COVID-19 vaccines and clear criteria for their potential authorization or approval in the US."
Every day, we follow the science in seeking safe & effective solutions for patients. Along with our fellow Biopharma leaders, #WeStandWithScience in the development of a potential #COVID19 vaccine. We are also committed to broad & equitable access of the vaccine around the world. pic.twitter.com/DMJw2VnZUh
— AstraZenecaUS (@AstraZenecaUS) September 8, 2020
Chief executives of nine drug companies, including AstraZeneca, BioNTech, Moderna, Pfizer, Novavax, Sanofi, GlaxoSmithKline, Johnson & Johnson, and Merck vowed not to rush a vaccine.
The pledge is intended to "help ensure public confidence in the rigorous scientific and regulatory process by which COVID-19 vaccines are evaluated and may ultimately be approved."
Scientists are evaluating the public health impact of rushing a vaccine and the potential safety concerns, against the effect of delaying this highly anticipated treatment.
But, they point out that temporary halts are common in vaccine trials.
Meanwhile, two other vaccines are in stage-three trials in the US.
Just this week, President Trump said there could be a vaccine before Election Day. "So, we're going to have a vaccine very soon. Maybe even before a very special date," he stated.
STAY UP TO DATE WITH THE FREE CBN NEWS APP
Click Here Get the App with Special Alerts on Breaking News and Top Stories
Your health is important. Do you have questions about nutrition, weight loss, boosting immunity or medicine? Learn more here!
Need prayer? We’re available 24/7. Call (800) 700-7000 or request prayer.
Learn why Truth Matters at CBN News.Did you know?
God is everywhere—even in the news. That’s why we view every news story through the lens of faith. We are committed to delivering quality independent Christian journalism you can trust. But it takes a lot of hard work, time, and money to do what we do. Help us continue to be a voice for truth in the media by supporting CBN News for as little as $1.











 Support CBN News
Support CBN News